Sp3 (diamond) carbon-based materials hold an eminent position in electrochemistry, a discipline which has a range of applications from laboratory to industrial scale and from analytical science to energy storage. Typically, their conductivity is achieved by incorporation of boron atoms in the diamond lattice during the chemical vapor deposition (CVD) preparation procedure. The conditions during CVD strongly influence structural, electronic, optical, spectroscopic, and electrochemical properties of prepared boron doped diamond (BDD) [1].
Electrochemical applications of BDD electrodes are intertwined to the water decomposition reaction (1) at high anodic potential enabled by the high oxygen overvoltage (E0 (•OH/H2O) = +2.8 V vs. SHE) at the diamond surface.
H2O → HO•+ H++e- (1)
Hydroxyl radicals (HO•), formed at the diamond surface, are powerful oxidizing agents capable of anodic 1/ incineration of a wide range of organic compounds for water disinfection, or 2/ production of intermediate reactive compounds for electrosynthesis and electrocatalysis. Applications in electroanalysis rely on direct oxidation or reduction of analytes within the wide potential window of BDD electrodes. The importance of surface morphology and pre-treatment on electron transfer rates and adsorption proclivity of redox active species is stressed frequently [2].
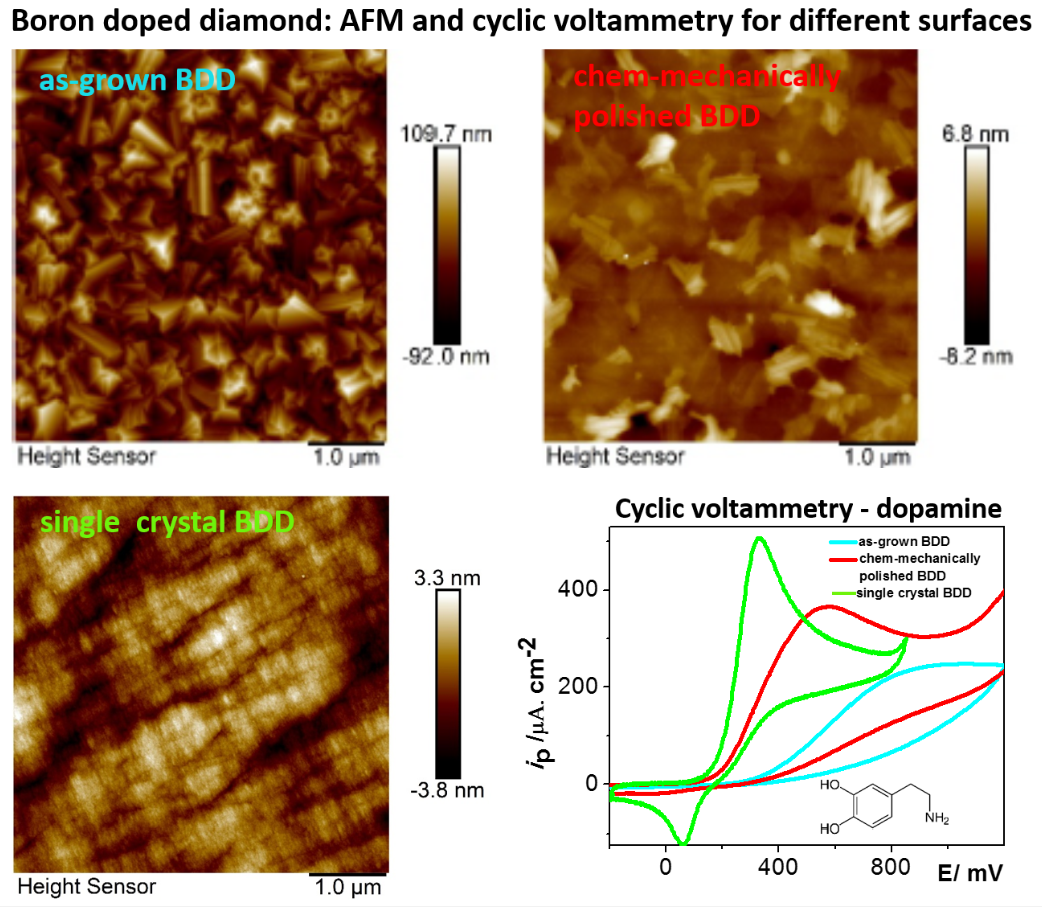
UNESCO Laboratory of Environmental Electrochemistry has built a strong collaboration with the Materials for Nanosystems and Biointerfaces (MNB) research group at the Institute of Physics of the Czech Academy of Sciences, who has acquired unique know-how on the synthesis and characterization of BDD coatings. Their last achievements include on one hand synthesis of structured BDD layers including porous BDD [3], but on the other hand preparation of relatively smooth BDD surfaces including chem-mechanically polished BDD or single crystal BDD differing in crystal orientation. The preliminary results indicate significantly enhanced electron-transfer rates on some of the surfaces and changes in adsorption proclivity for simple organic molecules.
Within this context, the proposed project aims to study the interplay between the surface and bulk properties of BDD and electrochemical performance. The objectives include:
This project is focused on the preparation and systemic characterization of conductive diamond-based materials. A transition of the newly acquired knowledge to application fields of these materials as anodes in incineration of organic compounds, electrosynthesis or as electrodes in electroanalysis is a desired outcome, for which the project solution will create a strong background.
The project will be solved in close cooperation with Institute of Physics (MNB research group, HiLASE centrum (Dr. Andy Taylor)) and Institute of Biophysics (Prof. Miroslav Fojta). High publication activity of all of the participating laboratories, including joint articles, and extensive experience with international collaboration together with enthusiasm and high professionalism of the staff will ensure successful outcomes of the project.
[1] 10.1039/c7cs00757d
[2] 10.1016/j.aca.2019.05.041
[3] 10.1016/j.electacta.2019.135025
Funding and project approval:
Co-founding resources:
Department of Analytical Chemistry
Required application materials:
How to submit application materials:
Please send email with the required application materials to Assoc. Prof. Karolina Schwarzová: karolina.schwarzova@natur.cuni.cz, Faculty of Science, Charles University, Prague.
Application deadline:
The application deadline is July 23, 2021.
More information:
For more information please visit the webpage of the JUNIOR Fund project of the Charles University.